DOW CORNING RUPTURED IMPLANTS
Posted On: October 14, 2011 Author: The Office of Dr. Stuart Linder Posted In: Breast Implants, Breast Revision, Breast topics, Home
We see patients who present with implants from the Dow Corning era. Those are implants placed well over 20 to 35 years ago. These implants are obviously no longer allowed on the market. The company is no longer producing these implants. The Dow Corning implants were smooth shelled implants that had a Dacron patch on the posterior wall.�
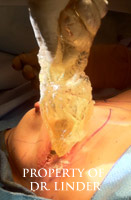 This photo is an example indicates a patient who recently had explantation, open capsulectomy and removal of ruptured silicone implant capsule material silicone granuloma extraction, reconstruction with Allergan cohesive gel style 20 implants. The patient had the silicone loose implant material completely exuded and removed through her previous inframammary incision. Subsequently, the pocket was irrigated with antibiotic solution. Total exenteration using electrocautery was required to remove the thick and hard shell of the calcified silicone material circumferentially in the pocket from the infraclavicular parasternal ridge along the anterior axillary line, along the lateral pectoralis minor muscle to the inframammary fold. The posterior chest wall capsule shell was also removed.�
This photo is an example indicates a patient who recently had explantation, open capsulectomy and removal of ruptured silicone implant capsule material silicone granuloma extraction, reconstruction with Allergan cohesive gel style 20 implants. The patient had the silicone loose implant material completely exuded and removed through her previous inframammary incision. Subsequently, the pocket was irrigated with antibiotic solution. Total exenteration using electrocautery was required to remove the thick and hard shell of the calcified silicone material circumferentially in the pocket from the infraclavicular parasternal ridge along the anterior axillary line, along the lateral pectoralis minor muscle to the inframammary fold. The posterior chest wall capsule shell was also removed.�
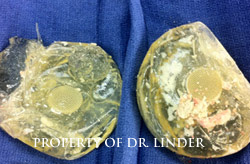 Note, the pictures of the implant showing complete loss of integrity, probably well over 15 years ago had been ruptured. Notice the thick, hard calcified shell of the entire exenterated capsule that has been removed, that this is silicone that has bled into the tissue through the capsule and has now caused calcification, hardening and actually loose calcified silicone material within the pocket.�
Note, the pictures of the implant showing complete loss of integrity, probably well over 15 years ago had been ruptured. Notice the thick, hard calcified shell of the entire exenterated capsule that has been removed, that this is silicone that has bled into the tissue through the capsule and has now caused calcification, hardening and actually loose calcified silicone material within the pocket.�
All Dow Corning implants should be removed. The integrity of all these shells probably last less than 10 years. These should be removed as soon as possible, the pockets should be cleaned and all scar tissue and capsule should be exenterated. Reconstruction with saline or silicone implants can be performed.